Electroplating is an electrochemical process used to deposit metals on other materials. In this article, we’ll explain what electroplating is, how it works, and where it’s used.
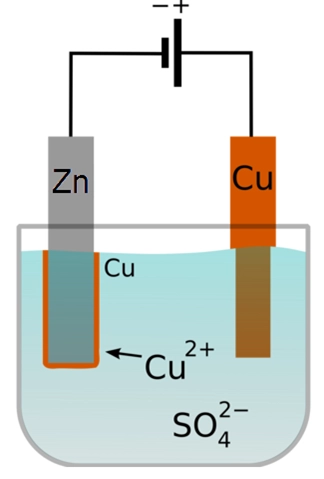
Electroplating is a chemical process that uses electricity to coat metal objects with another material. It’s often used in manufacturing to create decorative finishes for jewelry, coins, and other items.
What Is Electroplating?
Electroplating is the process of applying a thin layer of metal onto an object. This process involves passing a current through a solution containing ions of the metal being deposited. As the ions move toward the cathode (the negative terminal), they combine with electrons to form atoms of the metal. These atoms then bond to the surface of the object.
How Does It Work?
To understand how electroplating works, let’s start by looking at the chemical equation for the reaction between copper and sodium hydroxide. Copper(II) sulfate is dissolved in water, and sodium hydroxide is added to the solution. Sodium hydroxide reacts with the copper ions to produce copper(I) oxide and hydrogen gas. The copper(I) oxide dissolves in the solution, forming copper(II) hydroxide. The hydrogen gas bubbles away, leaving behind a solid coating of copper on the surface of the object being plated.
Where Can I Find Out More About Electroplating?
If you’re interested in learning more about electroplating, check out our articles on the subject. You can also find out more about electroplating on Wikipedia.
Where Can You Find It Used Today?
Electroplating is often used in manufacturing processes to add metal coatings to various products. These products range from jewelry to medical devices to automobiles.

Pingback: Aluminium Building Coating Options Available Today - RAI Blog
manuscripts significantly